1. Characteristics of LNG
LNG is liquefied natural gas. The liquefaction process first requires treatment to remove impurities such as nitrogen, carbon dioxide, hydrogen sulfide, other sulfur compounds and water. Then the natural gas, which is mainly composed of methane, will liquefy at a temperature of about -160oC, ready for storage and transportation. LNG occupies only 1/600 of the volume required for an equivalent amount of natural gas at room temperature and atmospheric pressure.
In general, LNG is a supercooled, non-toxic, non-corrosive substance that is transported and stored at low pressure. Instead of being compressed, it is cooled to liquefy, allowing LNG to be an efficient and economical for transporting large volumes over long distances. LNG by itself is of little danger when stored in tanks, pipelines and equipment designed for use in supercooled conditions. However, once LNG vaporizes, if not properly and safely controlled it can become flammable and explosive under certain conditions.
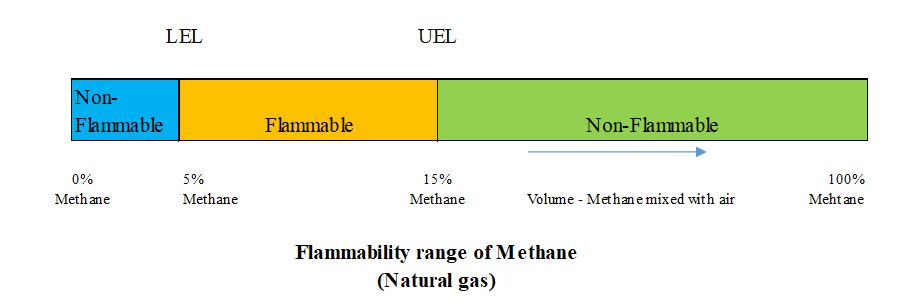
Flammability range is the range between the minimum and maximum concentrations of LNG vapor (% by volume), where air and LNG vapor form an explosive mixture. The figure above shows that the flammability of methane (the main component of LNG vapor) has upper and lower limits of 15% and 5% by volume mixed with air. When the concentration of methane exceeds the upper limit, it cannot burn because there is too little oxygen; for example in a closed and secure storage tank the concentration is approximately 100% methane. When the methane concentration is less than the lower limit, it also cannot burn because there is too little methane. A specific example is a small leak of LNG in a well-ventilated area, where the methane vapor will quickly mix with the air and disperse to less than 5% concentration. As such, methane will only be combustible if the mixture to air ratio is within the flammability range (5-15%). The usual danger is that it will ignite on contact with an open flame or spark.
The auto-ignition temperature is the lowest temperature at which a flammable vapor will ignite spontaneously, after only a few minutes of exposure, without the need for an ignition source. The higher the temperature is, the shorter the exposure time. Tested for methane generated from LNG, with a mixture ratio of about 10% methane in air (in the 5-15% flammability range) and under atmospheric pressure with an auto-ignition temperature above 540°C . In the event of an LNG spill on the ground or on water, if the flammable vapor is not exposed to an ignition source (flame, spark, or heat source of 540 °C or higher), the vapor will diffuse into the atmosphere and not cause fire and explosion.
Comparison table of fuel characteristics:
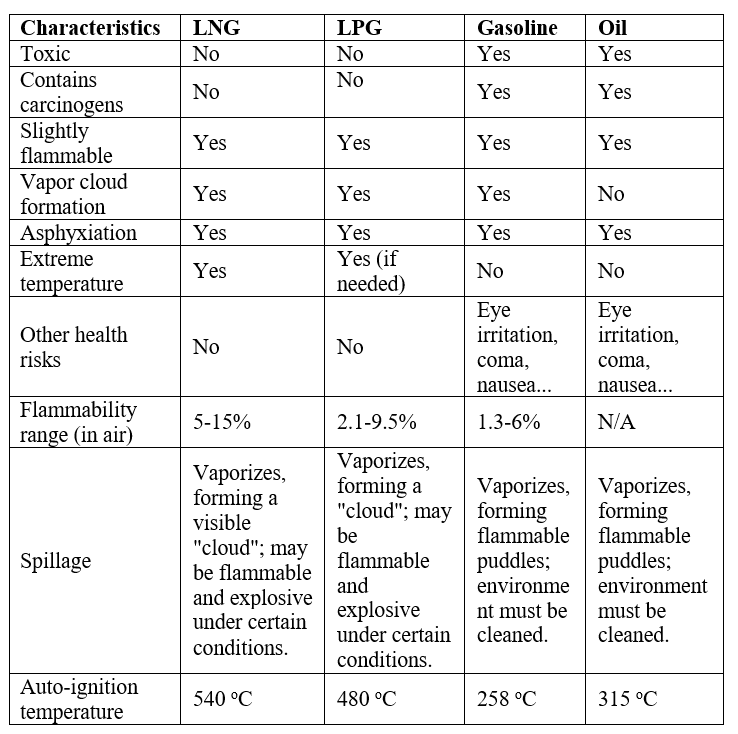
It can be seen from the above table that the flammability range of LNG has the highest Lower Explosive Limit (LEL) compared to LPG and gasoline, that is, more LNG vapor is needed to cause an explosion. Moreover, the spontaneous combustion temperature of LNG is also the highest compared to other fuels. In addition, LNG vapor is not as flammable as other conventional fuels and diffuses more easily into the air, meaning potential hazards will have a shorter lifetime than other fuels.
Thus, to consider whether LNG is a hazard, we need to understand the characteristics of LNG, as well as the necessary conditions for potential hazards to occur.
2. Hazards from LNG
a. Fire
LNG vapor, mainly methane, burns only in a narrow range of 5% to 15% methane-air mixtures. If the methane concentration is lower than 5%, it cannot burn because there is not enough fuel. If the fuel concentration is higher than 15%, it cannot burn because there is not enough oxygen. For LNG to burn, it must escape, vaporize, mix with air in proportions within the flammability range, and come into contact with an ignition source.
b. Explosion
Explosion occurs when a substance rapidly changes its chemical state or is released uncontrollably from a pressurized state. Explosion is an unlikely hazard with LNG operations. LNG itself in liquid form will not explode while in the tanks, because it is stored at -160 oC and low pressure. LNG cannot explode without high pressure or an enclosed space. An explosion due to LNG vapor can only occur if the following conditions simultaneously occur: vapor concentration is in the explosive range, vapor is in an enclosed space, and there is a source of ignition.
c. Vapor cloud
As LNG leaks into the environment, it begins to heat up, vaporizing back into a gaseous state. At first, the gas is colder and heavier than the surrounding air, which creates a fog (vapor cloud) above the liquid. Then, as the gas heats up, it mixes with the surrounding air and begins to disperse. The vapor cloud only ignites if it encounters an ignition source and methane vapor concentration is within the flammability range.
d. Super cold liquid
If LNG is released, direct human contact with the liquid at cathode temperatures will freeze the point of contact. Therefore, all personnel working with LNG must wear gloves, masks and protective clothing to protect from injury when entering hazardous areas. Furthermore, dike systems around LNG storage tanks must be designed to hold up to 110% of the tank’s contents.
e. Rapid Phase Transition (RPT)
When LNG comes into contact with water, because it is lighter it will float to the top until vaporizing. If large amounts of LNG spill over water, direct contact with LNG water can lead to a sudden burst of heat transfer and LNG vaporization. This rapid phase transition can cause small to moderate explosions, and is large enough to potentially damage light and medium structures. RPT hazards in LNG transportation can be considered minimal, due to the relatively small volume of LNG and very low proportion of LNG exposed to large bodies of water such as rivers, lakes or seas. Studies have shown that to release the energy that can cause RPT, the leakage rate must exceed 38 m3/minute.
f. Asphyxiation
As with other fuels, LNG vapor can cause asphyxiation when stored and used in locations with limited or no oxygen.
3. Safety in using LNG
After understanding the characteristics as well as potential hazards, how to ensure safe use of LNG?
Safety in the use of LNG is ensured by four “defense walls”, which provide multiple layers of protection for the safety of personnel working directly, as well as adjacent structures. The containment unit is the first and foremost line of defense used to store LNG products. This first layer of protection requires appropriate materials, as well as proper engineering design for LNG storage facilities
The second “defense wall” is to ensure that if a leak or spill occurs at the construction site, LNG can be completely contained and isolated from neighboring facilities. This defense requires construction of a dike to prevent spills, arranged around LNG storage tanks, vaporizers and related equipment. In addition, at points where there is a high probability of leakage or spillage, a collection system must be in place.
The third “defense wall” is protection systems. Primary objective is to reduce the frequency and amount of LNG released and to prevent harm from potential hazards involved, such as explosions, fires, etc. This defense requires technologies such as fire & gas detection warning system, Emergency Shutdown System (ESD), fire fighting system…. Furthermore, necessary procedures and instructions on operation, training and periodic maintenance are required to protect people, property and environment in the event of an incident.
The last “defense wall” is a safe distance. LNG facilities are required to comply with regulations to maintain a safe distance between LNG storage equipment as well as separate from surrounding facilities.
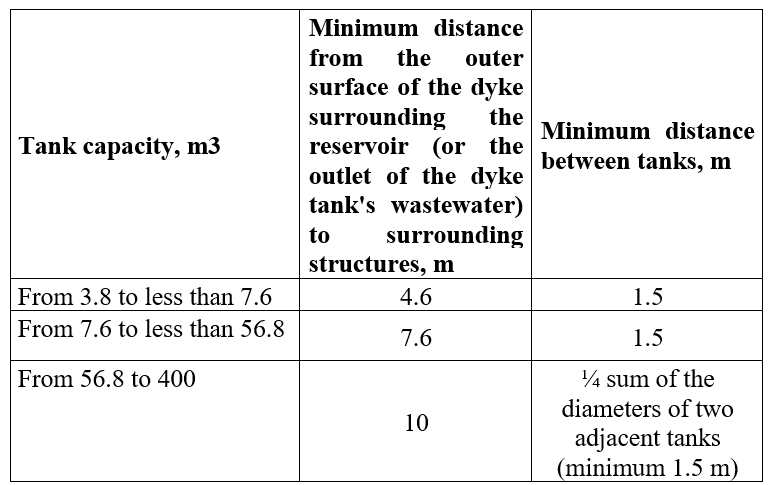
According to the standard TCVN 11278:2015, the distance between tanks to each other and to neighboring works is as follows:
References:
- National standard TCVN 11278:2015 Liquefied natural gas LNG – Equipment and installation system – LNG storage with capacity up to 200 Tons;
- NFPA 59A Standard for the Production, Storage, and Handling of Liquefied Natural Gas (LNG);
- ISO 16924:2016 LNG station for fuelling vehicles;
- https://www.youtube.com/watch?v=h-EY82cVKuA
- Article: LNG safety and security, Michelle Michot Foss, Rice University.





